A team of scientists led by KOO Sagang from the Seoul National University and the Center for Nanoparticle Research within the Institute for Basic Science Center (IBS), in collaboration with researchers from Korea Institute of Science and Technology (KIST) and the Seoul National University, developed a new solution for treating rheumatoid arthritis (RA).
Their study is published in Nature Nanotechnology.
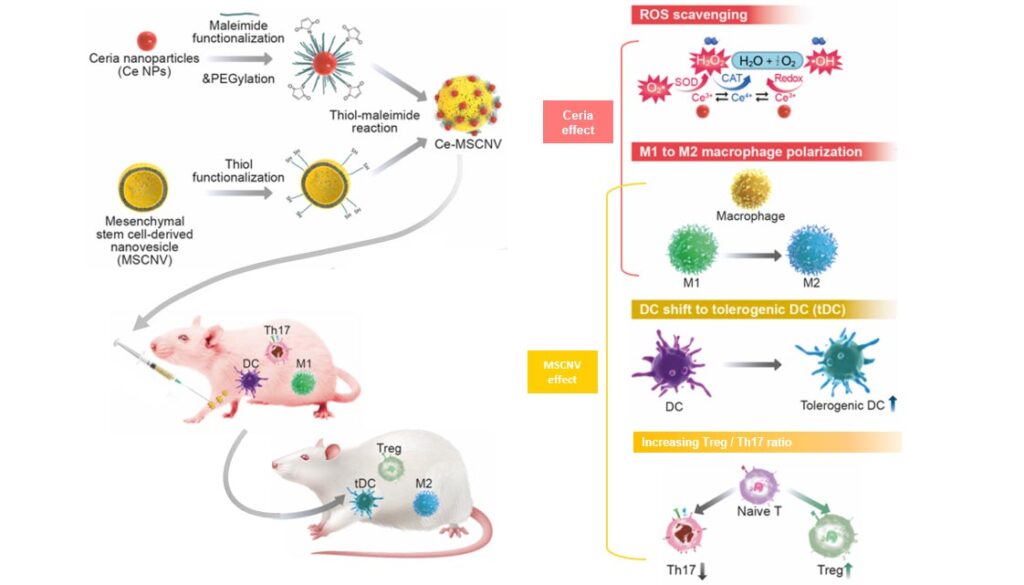
The scientists developed a nanoparticle-based platform for treating RA that comprises ceria nanoparticles (Ce NPs) immobilized onto mesenchymal stem cell-derived nanovesicles (MSCNVs).
RA is a chronic, inflammatory autoimmune disease for which there is no cure. The disease triggers a mix of troublesome symptoms and mechanisms, such as inflamed joints, the production of harmful cytokines, and immune system imbalances, which work together to create a relentless cycle of worsening symptoms. Moreover, the authors pointed out, Although the precise aetiological causes of RA remain unclear, it has been established that those factors act cooperatively, creating a vicious cycle with inevitable effects on the occurrence and progression of RA.”
So, while targeting some of the factors involved can provide short-term relief, other contributory factors remain unresolved, leading to a frustrating cycle of remission and flare-ups. First line treatment typically involves the use of anti-inflammatory drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and biological agents, but these “focus merely on symptom alleviation,” the investigators stated, and may only be temporarily effective.
In essence, the ideal treatment for RA should not only provide immediate relief from inflammation and symptoms but also address the root cause by restoring the immune system to its normal, balanced state. “Thus, an ideal RA treatment should involve the restoration of normal immune functionality and the immediate suppression of inflammatory responses and symptoms,” the team noted. “… multifunctional intervention systems that can hinder multiple pathogenic factors for exhaustive RA treatment are highly demanded.”
The ability to restore the immune system to its healthy state remains one of the major hurdles in RA treatment. “ … the balance between T helper 17 (TH17) cells and Treg cells has been known to be critical in the pathogenesis of autoimmune and inflammatory diseases, especially in RA,” the investigators pointed out. Without this balance, the body may not be able to control the continuous production of harmful substances like reactive oxygen species (ROS) and inflammatory cytokines, leading to persistent inflammation and discomfort.
The new platform developed by Koo and colleagues involves immobilizing ceria nanoparticles onto mesenchymal stem cell-derived nanovesicles. Both of these components can hinder different pathogenic factors, allowing them to work individually, but also cooperatively. Ce NPs can scavenge the overproduced ROS in RA-inflicted knee joints. They also induce polarization of M1 macrophages into M2, achieving immediate relief of inflammation and symptoms. MSCNVs deliver immunomodulatory cytokines, which turn dendritic cells (DC) into tolerogenic dendritic cells (tDCs). This generates regulatory T cells for long-term immune tolerance. The combination of both parts in the Ce-MSCNV nanoparticles aims to bridge both innate and adaptive immunity to achieve both short-term pain relief, as well as convert the tissue environment into an immune-tolerant state to prevent the recurrence of symptoms.
The authors further explained, “These two key elements (Ce NPs and MSCNVs) of Ce-MSCNVs work both individually and cooperatively to create a synergy, providing rapid treatment of the damaged joints, modulation of the immune cells and a rebalancing of the disturbed TH17/Treg cell ratio.”
For their reported study the researchers demonstrated the efficacy of the nanohybrid therapeutic approach in a collagen-induced arthritis mouse model. “Our aim was to break the vicious cycle of RA by creating a treatment invulnerable to the complications incurred by synovial inflammation, detrimental cytokines, and disturbed immune tolerance,” they wrote. The in vivo tests showed that the Ce-MSCNV system was able to comprehensively treat and prevent RA in the mice by simultaneously relieving the immediate and restoring T cell immunity. Supporting data suggest that improvement in conditions can be achieved after only a single-dose treatment. “The outstanding efficacy of Ce-MSCNVs was verified not only by the immediate and continuous therapeutic effects on CIA but also by the meaningful achievement with a single-dose treatment and the successful prevention of CIA,” the scientists pointed out.
First author KOO Sagang stated, “One of the hardest decisions in intractable disease therapy is determining how long the treatment should be carried on. For RA, it would not be appropriate to stop treatment just because the target marker is stabilized. A safer indicator should be that the innate and adaptive components of the collapsed immune system are normalized to protect the body.”
The scientists suggest that their study proves the potential of a hybrid nanoparticle system for the comprehensive treatment of autoimmune disease and modulation of the immune system.